
Chemistry, 22.10.2019 01:00 maggiestevens5321
Calculate the amount of heat transferred when 24.0g of ch3oh(g) is decomposed by this reaction at constant pressure. for a given sample of ch3oh , the enthalpy change during the reaction is 82.6kj . what mass of methane gas is produced? how many kilojoules of heat are released when 38.7g of ch4(g) reacts completely with o2(g) to form ch3oh(g) at constant pressure?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, shreyapatel2004
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
Calculate the amount of heat transferred when 24.0g of ch3oh(g) is decomposed by this reaction at co...
Questions in other subjects:

Mathematics, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31


Health, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31

Biology, 14.01.2020 19:31


Mathematics, 14.01.2020 19:31

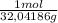 = 0,749 moles of CH₃OH(g)
= 0,749 moles of CH₃OH(g)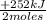 = 94,4 kJ
= 94,4 kJ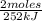 = 0,656 moles of CH₄
= 0,656 moles of CH₄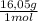 = 10,5 g of CH₄
= 10,5 g of CH₄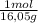 = 2,41 moles of CH₄(g)
= 2,41 moles of CH₄(g)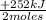 = 304 kJ
= 304 kJ


