
Chemistry, 21.10.2019 23:30 rachelsweeney10
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then adds iron powder to the conical flask at once. then, she stirs it briskly to initiate and facilitate the reaction between copper sulfate and iron powder. while stirring continuously, vanessa observes that the blue color of copper sulfate solution is fading substantially. moreover, she also observes the deposition of a reddish-brown mass at the bottom of the flask.
finally, a stage is reached where the solution becomes pale green in color. on further stirring of the solution, there is no change in the intensity of color. a distinct reddish-brown layer is deposited in the conical flask. however, on weighing the reaction mixture, she finds that the mass of the reaction mixture remains unchanged at 215.4 g. the reddish-brown layer is then separated from the pale green solution and allowed to dry in an oven. the moisture is completely evaporated, and the dried layer is weighed. its weight is found to be 63.5 g.
what is the mass of required iron powder and the formed iron sulfate in the chemical reaction?
a
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
b
the mass of iron powder required to complete the reaction was 63.5 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
c
the mass of iron powder required to complete the reaction was 159.6 g, while the mass of iron sulfate formed in the reaction was 63.5 g.
d
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 159.6 g.
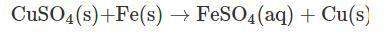

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then...
Questions in other subjects:


Biology, 02.09.2019 06:50

English, 02.09.2019 06:50

Mathematics, 02.09.2019 06:50

Mathematics, 02.09.2019 06:50


Mathematics, 02.09.2019 06:50

Biology, 02.09.2019 06:50


Chemistry, 02.09.2019 06:50



