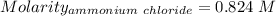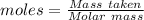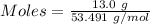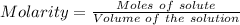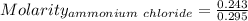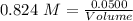Asolution is created by dissolving 13.0 grams of ammonium chloride in enough water to make 295 ml of solution. how many moles of ammonium chloride are present in the resulting solution? =0.243 moleswhen thinking about the amount of solute present in a solution, chemists report the concentration or molarity of the solution. molarity is calculated as moles of solute per liter of solution. what is the molarity of the solution described above? =0.824 mto carry out a particular reaction, you determine that you need 0.0500 moles of ammonium chloride. what volume of the solution described above will you need to complete the reaction without any leftover nh4cl? ml of solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
Asolution is created by dissolving 13.0 grams of ammonium chloride in enough water to make 295 ml of...
Questions in other subjects:






History, 25.03.2020 17:49


Chemistry, 25.03.2020 17:49