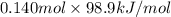Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
Calculate the amount of energy in the form of heat that is produced when a volume of 3.43 l of so2(g...
Questions in other subjects:


Social Studies, 13.12.2019 00:31








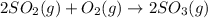
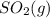 and
and 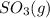 are as follows.
are as follows.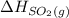 = -296.8 kJ/mol
= -296.8 kJ/mol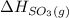 = -395.7 kJ/mol
= -395.7 kJ/mol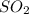 =
= 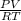
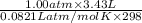 (as 1 bar = 1 atm (approximately))
(as 1 bar = 1 atm (approximately))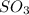 are also 0.140.
are also 0.140.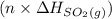 and
and 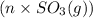
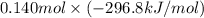 and
and 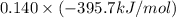 respectively.
respectively.![0.140 \times [-296.8 kJ/mol - (-395.7 kJ/mol)]](/tpl/images/0333/5672/320d5.png)