
Chemistry, 19.10.2019 04:30 lapointayyy6388
What is the vapor pressure (in kpa) of ethanol, ch3ch2oh, over a solution which is composed of 18.00 ml of ethanol and 12.55 g of benzoic acid, c6h5cooh, at 35ºc ?
enter your number with two digits past the decimal.
•pºethanol at 35ºc = 13.693 kpa
•density of ethanol = 0.789 g/mol, molar mass of ethanol = 46.07
•molar mass of benzoic acid = 122.12 g/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
What is the vapor pressure (in kpa) of ethanol, ch3ch2oh, over a solution which is composed of 18.00...
Questions in other subjects:

Mathematics, 17.07.2020 01:01






Geography, 17.07.2020 01:01



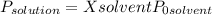 (1)
(1)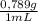 ×
×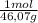 = 0,3083 mol Ethanol.
= 0,3083 mol Ethanol.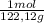 = 0,1028 mol benzoic acid.
= 0,1028 mol benzoic acid.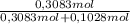 = 0,7499
= 0,7499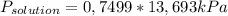 = 10,27 kPa
= 10,27 kPa

