
Chemistry, 19.10.2019 00:30 hannahbannana98
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthalein as the indicator. in the reaction of oxalic acid with naoh, every 1 mole of oxalic acid is deprotonated by 2 moles of naoh. the molecular weight of oxalic acid is 90.03 g/mol and you use 0.60 g in 50 ml water for the titration. you use 15.20 ml of your naoh solution in order to reach the endpoint of the titration. what is the concentration of your naoh solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, issachickadi
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1
You know the right answer?
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthale...
Questions in other subjects:





Computers and Technology, 03.01.2020 03:31


Social Studies, 03.01.2020 03:31

Business, 03.01.2020 03:31


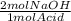 = 0.0133 mol NaOH
= 0.0133 mol NaOH

