
Chemistry, 19.10.2019 00:20 alexvillaa121
Ph is a logarithmic scale used to indicate the hydrogen ion concentration, [h+], of a solution: ph=−log[h+] due to the autoionization of water, in any aqueous solution, the hydrogen ion concentration and the hydroxide ion concentration, [oh−], are related to each other by the kw of water: kw=[h+][oh−]=1.00×10^−14 where 1.00×10^−14 is the value at approximately 297 k. based on this relation, the ph and poh are also related to each other as 14.00=ph+poh. the temperature for each solution is carried out at approximately 297 k where kw=1.00×10^−14. part a) 0.35 g of hydrogen chloride (hcl) is dissolved in water to make 2.5 l of solution. what is the ph of the resulting hydrochloric acid solution? express the ph numerically to two decimal places.
part b) 0.80 g of sodium hydroxide (naoh) pellets are dissolved in water to make 2.0 l of solution. what is the ph of this solution?
express the ph numerically to two decimal places.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Ph is a logarithmic scale used to indicate the hydrogen ion concentration, [h+], of a solution: ph=...
Questions in other subjects:

Mathematics, 02.03.2021 20:40


Mathematics, 02.03.2021 20:40


Mathematics, 02.03.2021 20:40


Mathematics, 02.03.2021 20:40

Mathematics, 02.03.2021 20:40


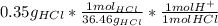 = 9.60*10⁻³ mol H⁺
= 9.60*10⁻³ mol H⁺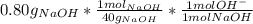 = 0.02 mol OH⁻
= 0.02 mol OH⁻

