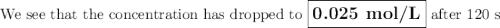Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, officialgraciela67
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1


Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
The decomposition of n2o5(g) —> no2(g) + no3(g) proceeds as a first order reaction with a half l...
Questions in other subjects:

Biology, 03.10.2019 08:30



Mathematics, 03.10.2019 08:30

History, 03.10.2019 08:30

Mathematics, 03.10.2019 08:30


Mathematics, 03.10.2019 08:30

History, 03.10.2019 08:30

Mathematics, 03.10.2019 08:30

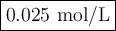
![\begin{array}{crcc}\textbf{No. of} && \textbf{Fraction} & \\\textbf{half-lives} & \textbf{t/s} & \textbf{remaining} &\rm \mathbf{{[N_{2}O_{5}] /(mol/L)}}\\0 & 0 & 1 & 0.400\\1 & 30.0 & 1/2 & 0.200\\2 & 60.0 & 1/4 & 0.100\\3 & 90.0 &1/8 & 0.050\\4 & 120.0 & 1/16 & 0.025\\5& 150.0 & 1/32 & 0.012\\\end{array}](/tpl/images/0332/8503/2bb43.png)