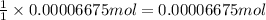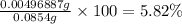Chemistry, 18.10.2019 22:00 hei40563273
In part 2 of the experiment, you will be analyzing a sample of household bleach. a 0.0854 g sample of household bleach is completely reacted with ki(s). the resulting solution is then titrated with 0.150 m nas2o3 solution. 0.890 ml of the solution is required to reach the colorless endpoint. what is the mass percent of naocl (mm = 74.44 g/mole) in the bleach? a. 30.1% b. 96.5% c. 2.23% d. 5.82%

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, sillslola816oxb5h7
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1

Chemistry, 23.06.2019 01:10, minasotpen1253
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
In part 2 of the experiment, you will be analyzing a sample of household bleach. a 0.0854 g sample o...
Questions in other subjects:


Mathematics, 13.10.2020 09:01



Biology, 13.10.2020 09:01

History, 13.10.2020 09:01

Mathematics, 13.10.2020 09:01



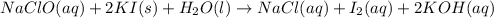 ..[1]
..[1]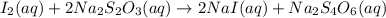 ..[2]
..[2]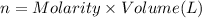
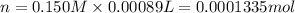
 .
. of
of