Chemistry, 18.10.2019 19:20 ineedhelp2285
Consider an ionic compound, mx, composed of generic metal m and generic, gaseous halogen x. the enthalpy of formation of mxis δh∘f=−427kj/mol. the enthalpy of sublimation of mis δhsub=135kj/mol. the ionization energy of mis ie=433kj/mol. the electron affinity of xis δhea=−307kj/mol. (refer to the hint). the bond energy of x2is be=175kj/mol. determine the lattice energy of mx.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

You know the right answer?
Consider an ionic compound, mx, composed of generic metal m and generic, gaseous halogen x. the enth...
Questions in other subjects:


Computers and Technology, 22.10.2019 00:00

Mathematics, 22.10.2019 00:00

English, 22.10.2019 00:00

Biology, 22.10.2019 00:00

Mathematics, 22.10.2019 00:00


English, 22.10.2019 00:00


 ....[1]
....[1] ....[2]
....[2]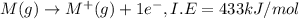 ....[3]
....[3]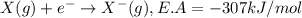 ....[4]
....[4] ....[5]
....[5] ....[6]
....[6]