
Chemistry, 18.10.2019 01:30 vanessam16
Question 8 of 8> 0 attempt 2 a chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. he has a 0.1284 m standard sodium hydroxide solution. he takes a 25.00 ml sample of the original acid solution and dilutes it to 250.0 ml. then, he takes a 10.00 ml sample of the dilute acid solution and titrates it with the standard solution. the endpoint was reached after the addition of 19.15 ml of the standard solution. what is the concentration of the original sulfuric acid solution? concentration: 025

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2


Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Question 8 of 8> 0 attempt 2 a chemist needs to determine the concentration of a sulfuric acid s...
Questions in other subjects:

History, 30.06.2019 03:00

Mathematics, 30.06.2019 03:00




Mathematics, 30.06.2019 03:00

Mathematics, 30.06.2019 03:00


Mathematics, 30.06.2019 03:00

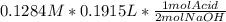 = 0.01229 mol H₂SO₄.
= 0.01229 mol H₂SO₄.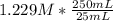 = 12.29 M
= 12.29 M

