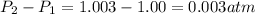Chemistry, 18.10.2019 01:30 Queenashley3232
At the normal melting point of nacl, 801 degrees c, its enthalpy of fusion is 28.8 kj / mol. the density of the solid is 2.165 g/cm^3 , and the density of the liquid is 1.733 g/ cm^3. what pressure increase is needed to raise the melting point by 1.00 degrees c?

Answers: 2


Other questions on the subject: Chemistry



You know the right answer?
At the normal melting point of nacl, 801 degrees c, its enthalpy of fusion is 28.8 kj / mol. the den...
Questions in other subjects:

Chemistry, 24.02.2021 07:00

Mathematics, 24.02.2021 07:00




Mathematics, 24.02.2021 07:00


Mathematics, 24.02.2021 07:00

Mathematics, 24.02.2021 07:00

Physics, 24.02.2021 07:00

![\ln(\frac{P_2}{P_1})=\frac{\Delta H}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0330/0903/5c76e.png)
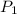 = initial pressure which is the pressure at normal boiling point = 1 atm
= initial pressure which is the pressure at normal boiling point = 1 atm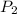 = final pressure = ?
= final pressure = ?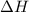 = Enthalpy change of the reaction = 28.8 kJ/mol = 28800 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy change of the reaction = 28.8 kJ/mol = 28800 J/mol (Conversion factor: 1 kJ = 1000 J)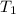 = initial temperature =
= initial temperature = ![801^oC=[801+273]K=1074K](/tpl/images/0330/0903/9b4bf.png)
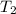 = final temperature =
= final temperature = ![(801+1.00)^oC=802.00=[802+273]K=1075K](/tpl/images/0330/0903/fe656.png)
![\ln(\frac{P_2}{1})=\frac{28800J/mol}{8.314J/mol.K}[\frac{1}{1074}-\frac{1}{1075}]\\\\\ln P_2=3\times 10^{-3}atm\\\\P_2=e^{3\times 10^{-3}}=1.003atm](/tpl/images/0330/0903/b8d88.png)