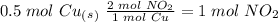Chemistry, 18.10.2019 03:30 GamerGirl15
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxide gas and liquid water. a. write the balanced equation for the reaction b. if there are 0.500 moles of copper metal present how many moles of nitric acid are required for the reaction? how many moles of nitrogen dioxide gas are formed?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, stephaniero6
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxid...
Questions in other subjects:

Chemistry, 05.05.2020 14:22

English, 05.05.2020 14:22

Mathematics, 05.05.2020 14:22


Mathematics, 05.05.2020 14:22



Mathematics, 05.05.2020 14:22

Biology, 05.05.2020 14:22

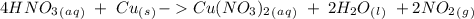
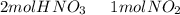
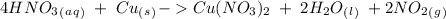
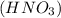 we have to use the molar ratio in the balence reaction:
we have to use the molar ratio in the balence reaction: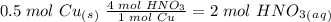
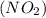 we have to follow the same logic:
we have to follow the same logic: