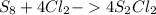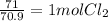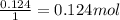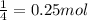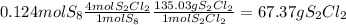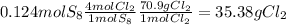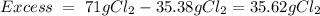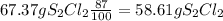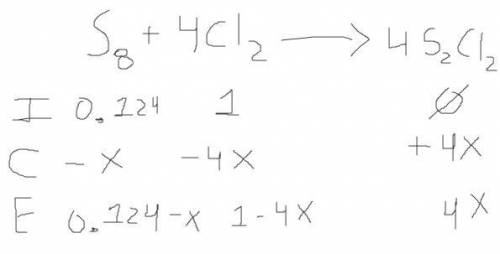Chemistry, 18.10.2019 03:30 ejfleck3655
6. write the ice chart for the reaction of 32.0 g of sulfur and 71.0 g of chlorine: s8 + 4 cl24s2cl2 after completing the chart give a. the mass of product b. the mass of agent in excess c. the mass produced if there is an 87.% yield

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 07:20, msladycie8831
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1

Chemistry, 23.06.2019 11:30, amiechap12
Which of the following is a possible formula unit? (2 points) select one: a. pbo b. li2b c. al2pb3 d. clo
Answers: 1
You know the right answer?
6. write the ice chart for the reaction of 32.0 g of sulfur and 71.0 g of chlorine: s8 + 4 cl24s2cl...
Questions in other subjects:

Mathematics, 06.07.2019 10:30

English, 06.07.2019 10:30




Mathematics, 06.07.2019 10:30

History, 06.07.2019 10:30

Mathematics, 06.07.2019 10:30

Biology, 06.07.2019 10:30

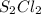
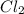
 and the reactives are consumed, so for
and the reactives are consumed, so for