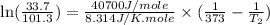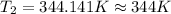Chemistry, 18.10.2019 01:20 dewayne5599
3. at sea level, the atmospheric pressure is 101.3 kpa. atop mount everest, the atmospheric pressure is 33.7 kpa. considering the normal boiling point of water (100 °c) and its heat of vaporization (40.7 kj/mol), at what temperature will water boil atop mount everest? m

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

You know the right answer?
3. at sea level, the atmospheric pressure is 101.3 kpa. atop mount everest, the atmospheric pressure...
Questions in other subjects:

Mathematics, 11.03.2021 17:20

Business, 11.03.2021 17:20

Mathematics, 11.03.2021 17:20

History, 11.03.2021 17:20

Mathematics, 11.03.2021 17:20


Mathematics, 11.03.2021 17:20


Mathematics, 11.03.2021 17:20

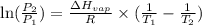
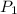 = atmospheric pressure at at sea level = 101.3 kPa
= atmospheric pressure at at sea level = 101.3 kPa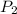 = atmospheric pressure at top mount everest = 33.7 kPa
= atmospheric pressure at top mount everest = 33.7 kPa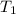 = normal boiling point of water =
= normal boiling point of water = 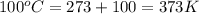
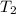 = temperature at top mount everest = ?
= temperature at top mount everest = ?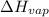 = heat of vaporization = 40.7 kJ/mole = 40700 J/mole
= heat of vaporization = 40.7 kJ/mole = 40700 J/mole