
Chemistry, 18.10.2019 01:10 jellybellyje
Which solvent will nacl, sodium chloride or table salt, will be more soluble in? nacl will be more soluble in kerosene because the non polar structure of kerosene allows the smaller lons to infiltrate the structure of the larger compound and therefore dissolve. nacl will be more soluble in kerosene because kerosene can dissolve all compounds. nacl will be equally soluble in both solvent. nal will be more soluble in water, because water is polar and can separate the cation and anion in the lonic compound, creating very strong ion dipole attractions

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, Luzperez09
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 08:40, alexisbcatlett14
Which statement can best be concluded from the ideal gas law?
Answers: 2


Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Which solvent will nacl, sodium chloride or table salt, will be more soluble in? nacl will be more...
Questions in other subjects:

English, 25.03.2020 23:02

English, 25.03.2020 23:02


History, 25.03.2020 23:02




Mathematics, 25.03.2020 23:02


Business, 25.03.2020 23:02

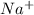 and
and 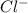 . As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.
. As a result, due to the opposite charges there will occur strong ion dipole attractions between these ions and water molecules.

