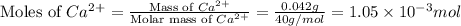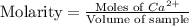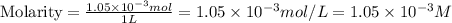Chemistry, 18.10.2019 00:30 hernsl0263
A) a water sample (density=1.00g/ml, s=0.28g/kg) contains ca(2+) at a concentration of 42 mg/kg. calculate the molarity of the ion.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
A) a water sample (density=1.00g/ml, s=0.28g/kg) contains ca(2+) at a concentration of 42 mg/kg. cal...
Questions in other subjects:

Mathematics, 01.09.2021 15:40

Mathematics, 01.09.2021 15:40

English, 01.09.2021 15:40




English, 01.09.2021 15:40

Mathematics, 01.09.2021 15:40

Mathematics, 01.09.2021 15:40

Biology, 01.09.2021 15:40

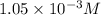
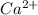 = 42 mg/kg
= 42 mg/kg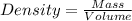
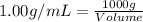
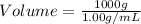
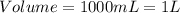 (1 L = 1000 mL)
(1 L = 1000 mL)