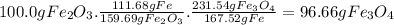calculate the weight of fe3o4 in 100.0g fe2o3.
given: molar mass of fe2o3 =159.69 g/mol...

Chemistry, 18.10.2019 01:00 CameronVand21
calculate the weight of fe3o4 in 100.0g fe2o3.
given: molar mass of fe2o3 =159.69 g/mol molar mass of fe3o4 = 231.54 g/mol hint: you need two conversion factors

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 16.10.2020 17:01


Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Spanish, 16.10.2020 17:01

Social Studies, 16.10.2020 17:01


Biology, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01