
Chemistry, 18.10.2019 01:00 19thomasar
The equilibrium constant kp for the reaction (ch3),cci (g) = (ch3),c=ch, (g) + hcl (g) is 3.45 at 500. k. (5.00 x 10k) calculate the value of kc at 500. k. for the same reaction, calculate the molar concentration of reactants and products at equilibrium if initially 1.00 mol of (ch3),cci was placed in a 5.00 l vessel.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
The equilibrium constant kp for the reaction (ch3),cci (g) = (ch3),c=ch, (g) + hcl (g) is 3.45 at 50...
Questions in other subjects:

Mathematics, 30.03.2020 17:37





History, 30.03.2020 17:37




 for the reaction is 6.32 and concentrations of
for the reaction is 6.32 and concentrations of 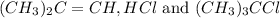 is 0.094 M, 0.094 M and 0.106 M respectively.
is 0.094 M, 0.094 M and 0.106 M respectively. is given by the formula:
is given by the formula: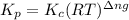
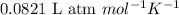
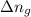 = change in number of moles of gas particles =
= change in number of moles of gas particles = 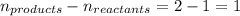
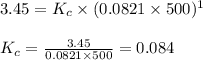
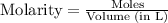
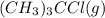 = 1.00 mol
= 1.00 mol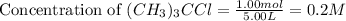

![K_c=\frac{[(CH_3)_2C=CH]\times [HCl]}{[(CH_3)_3CCl]}](/tpl/images/0329/9758/8a400.png)

![[(CH_3)_2C=CH]=x=0.094M](/tpl/images/0329/9758/5587a.png)
![[HCl]=x=0.094M](/tpl/images/0329/9758/e3b5a.png)
![[(CH_3)_3CCl]=(0.2-x)=(0.2-0.094)=0.106M](/tpl/images/0329/9758/364df.png)


