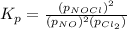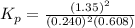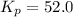Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 06:00, tytianadyson74
What volume of argon gas is equal to 1.60 grams of argon
Answers: 1

Chemistry, 23.06.2019 09:00, AdoNice
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground, its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3
You know the right answer?
The partial pressures in an equilibrium mixture of no, cl2, and noci at 500 k are as follows: pno =...
Questions in other subjects:

Mathematics, 25.01.2021 07:50

Mathematics, 25.01.2021 07:50

English, 25.01.2021 07:50


History, 25.01.2021 07:50





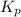 at temperature 500 K is 52.0
at temperature 500 K is 52.0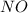 at equilibrium = 0.240 atm
at equilibrium = 0.240 atm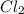 at equilibrium = 0.608 atm
at equilibrium = 0.608 atm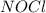 at equilibrium = 1.35 atm
at equilibrium = 1.35 atm