Which one of the following statements is true?
a. the enthalpy change for a reaction is inde...

Chemistry, 17.10.2019 19:00 unknown235
Which one of the following statements is true?
a. the enthalpy change for a reaction is independent of the state of the reactants and products.
b. enthalpy is an intensive property.
c. enthalpy is a state function.
d. h is the value of q measured under conditions of constant volume.
e. the enthalpy change of a reaction is the reciprocal of the δh of the reverse reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Cooldude3966
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2


Chemistry, 23.06.2019 02:50, giavanleer14
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Questions in other subjects:







Mathematics, 20.09.2020 04:01



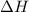 and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.
and its value depends on state functions like U, P and V. Therefore, change in enthalpy is also a state function.

