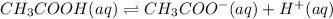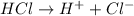Chemistry, 17.10.2019 17:30 happy121906
Choose the statement below that is true.
a. a weak acid solution consists of mostly non-ionized acid molecules.
b. the term "strong electrolyte" means that the substance is extremely reactive.
c. a strong acid solution consists of only partially ionized acid molecules.
d. the term "weak electrolyte" means that the substance is inert.
e. a molecular compound that does not ionize in solution is considered a strong electrolyte.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1
You know the right answer?
Choose the statement below that is true.
a. a weak acid solution consists of mostly non-ioniz...
a. a weak acid solution consists of mostly non-ioniz...
Questions in other subjects: