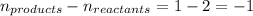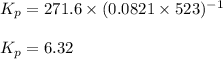Chemistry, 17.10.2019 18:00 pippalotta
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the other phosphorus compounds, coexist in equilibrium through the reaction
pcl3(g) + cl2(g) = pcl5(g)
at 250 ᵒc , an equilibrium mixture in a 25.0 l flask contains 0.105 g pcl5, 0.220 g pcl3 and 2.12 g of cl2. what are the values of
a) kc
b) kp for this reaction at 250 ᵒc ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinadhar
What are the percent by mass of copper in penny lab
Answers: 3

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the oth...
Questions in other subjects:


History, 29.10.2020 01:00

Mathematics, 29.10.2020 01:00


History, 29.10.2020 01:00

Geography, 29.10.2020 01:00


History, 29.10.2020 01:00


Physics, 29.10.2020 01:00

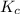 for the given reaction is 271.6
for the given reaction is 271.6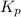 for the reaction is 6.32
for the reaction is 6.32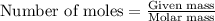 .....(1)
.....(1)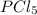 :
: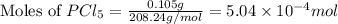
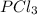 :
: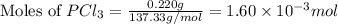
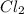 :
: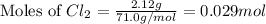
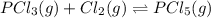
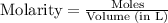
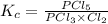
![[PCl_5]=\frac{5.04\times 10^{-4}mol}{25L}](/tpl/images/0328/9204/d1b89.png)
![[PCl_3]=\frac{1.60\times 10^{-3}mol}{25L}](/tpl/images/0328/9204/16be6.png)
![[Cl_2]=\frac{0.029mol}{25L}](/tpl/images/0328/9204/6d85a.png)
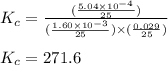
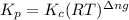
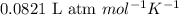
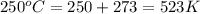
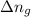 = change in number of moles of gas particles =
= change in number of moles of gas particles =