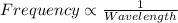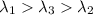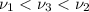One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of 2.12 10-10 m, and another type of electromagnetic radiation has photons with energy equal to 3.97 10-19 j/photon. identify each type of electromagnetic radiation. 107.1 mhz 2.12 10-10 m 3.97 10-19 j/photon fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light rank them in order of increasing photon energy and increasing frequency

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, janayflowers042
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1


Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of...
Questions in other subjects:

Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

Health, 13.10.2020 15:01

History, 13.10.2020 15:01

Health, 13.10.2020 15:01


Mathematics, 13.10.2020 15:01



Mathematics, 13.10.2020 15:01

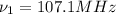

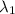

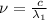
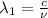

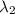
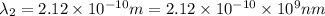
 (X-rays)
(X-rays)
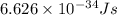

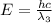


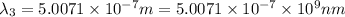
 (visible light)
(visible light)