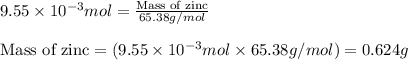Small quantites of hydrogen gas can be prepared in the laboratory by the addition of aqueous hydrochloric acid to metallic zinc. typically, the hydrogen gas is bubbled through water for collection and becomes saturated with water vapor. suppose 240. ml of hydrogen gas is collected at 30. c and has a total pressure of 1.032 atm by this process. what is the partial pressure of hydrogen gas in the sample? how many grams of zinc must have reacted to produce this quantity of hydrogen? (the vapor pressure of water is 32 torr at 30 c).

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, lexybellx3
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3


Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2
You know the right answer?
Small quantites of hydrogen gas can be prepared in the laboratory by the addition of aqueous hydroch...
Questions in other subjects:




Mathematics, 08.10.2019 20:00

History, 08.10.2019 20:00

History, 08.10.2019 20:00


History, 08.10.2019 20:00


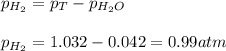
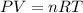
![30^oC=[30+273]K=303K](/tpl/images/0328/9260/fd4b3.png)
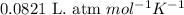
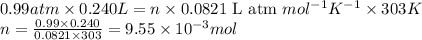
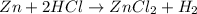
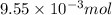 of hydrogen gas is produced from =
of hydrogen gas is produced from = 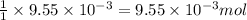 of zinc metal
of zinc metal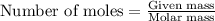
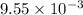 moles
moles