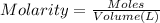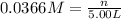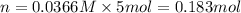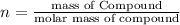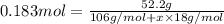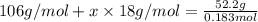Chemistry, 17.10.2019 16:20 edwardordonez66
Now that snape and dumbledore has taught you the finer points of hydration calculations they have a slightly more challenging problem for you. suppose you dissolve 52.2 g of na2co3 ∙ xh2o in enough water to make 5.00 l of solution. the final concentration of the solution was found to be 0.0366 m. determine the integer x in the hydrate: na2co3 ∙ xh2o. round your answer to the nearest integer.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 08:30, alexiasommers41
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1
You know the right answer?
Now that snape and dumbledore has taught you the finer points of hydration calculations they have a...
Questions in other subjects:




Mathematics, 03.11.2019 22:31

Mathematics, 03.11.2019 22:31

English, 03.11.2019 22:31