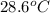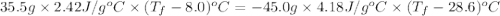Chemistry, 17.10.2019 06:00 steven2996
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (density = 1.0 g/ml) initially at 28.6 ∘c in an insulated beaker, what is the final temperature of the mixture, assuming that no heat is lost? (cetoh=2.42j/(g⋅∘

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:30, emfranco1
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 02:30, ineedhelp2285
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (den...
Questions in other subjects:

Mathematics, 15.06.2020 22:57


Mathematics, 15.06.2020 22:57

Business, 15.06.2020 22:57

History, 15.06.2020 22:57

Mathematics, 15.06.2020 22:57

Mathematics, 15.06.2020 22:57

Biology, 15.06.2020 22:57


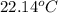
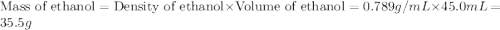
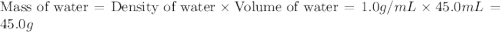
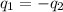
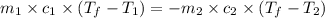
 = specific heat of ethanol =
= specific heat of ethanol = 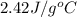
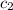 = specific heat of water =
= specific heat of water = 
 = mass of ethanol = 35.5 g
= mass of ethanol = 35.5 g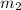 = mass of water = 45.0 g
= mass of water = 45.0 g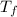 = final temperature of mixture = ?
= final temperature of mixture = ? = initial temperature of ethanol =
= initial temperature of ethanol = 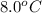
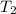 = initial temperature of water =
= initial temperature of water =