
Chemistry, 16.10.2019 21:20 sdwhitneyhillis
The rate of decay of a chemical involved in a reaction that is second order (bimolecular) in one reactant a is given by: = k [aj? -d[a/dt where k is the reaction rate coefficient. derive an expression for the half-life of a in terms of k and the concentration of a at time t-0 (ao)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


You know the right answer?
The rate of decay of a chemical involved in a reaction that is second order (bimolecular) in one rea...
Questions in other subjects:




Mathematics, 24.07.2019 15:30


Mathematics, 24.07.2019 15:30

Biology, 24.07.2019 15:30



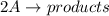
![\textrm{rate}=-\dfrac{\Delta[\textrm A]}{2\Delta t}=k[\textrm A]^2](/tpl/images/0326/2107/66a24.png)
![\dfrac{d[A]}{dt}=-k[A]^2](/tpl/images/0326/2107/6f64e.png)
![\int_{[A_t]}^{[A_0]}\frac{d[A]}{[A]^2}=-\int_{0}^{t}kdt](/tpl/images/0326/2107/7af1b.png)
![\dfrac{1}{[A]} = \dfrac{1}{[A]_0}+kt](/tpl/images/0326/2107/704d4.png)
![[A_t]](/tpl/images/0326/2107/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0326/2107/9a686.png) is the initial concentration
is the initial concentration![[A_t]=\frac{1}{2}\times [A_0]](/tpl/images/0326/2107/09fb2.png)
![t_{1/2}=\dfrac{1}{k[A_o]}](/tpl/images/0326/2107/4b6c5.png)


