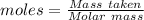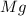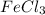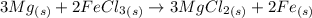Chemistry, 16.10.2019 06:00 jessica28757
Magnesium reacts with iron(iii) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3mg(s) + 2fecl3(s) → 3mgcl2(s) + 2fe(s) a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. what mass of magnesium chloride = 95.21 g/mol) is formed?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
Magnesium reacts with iron(iii) chloride to form magnesium chloride (which can be used in fireproofi...
Questions in other subjects:

Mathematics, 12.07.2019 19:30


Chemistry, 12.07.2019 19:30




Mathematics, 12.07.2019 19:30

Chemistry, 12.07.2019 19:30

Mathematics, 12.07.2019 19:30