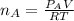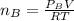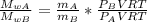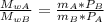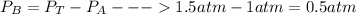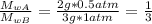Chemistry, 16.10.2019 04:30 atsuedem974
When 2 g of gaseous substance a are introduced into an initially evacuated flask kept at 25°c, the pressure is found to be 1 atm. three grams of gaseous substance b are then added to the 2g of a, and the pressure is now found to be 1.5 atm. assuming ideal gas behavior. calculating the ratio of molecular weights, that is, mwa /mwb

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, Queenquestion5967
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3


You know the right answer?
When 2 g of gaseous substance a are introduced into an initially evacuated flask kept at 25°c, the p...
Questions in other subjects:



Mathematics, 07.05.2020 00:02


Mathematics, 07.05.2020 00:02





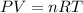
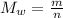
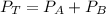
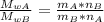 (equation 1)
(equation 1)