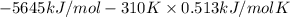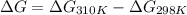Chemistry, 16.10.2019 04:00 Fatimaneedhelp
At 298 k the standard enthalpy of combustion of sucrose is -5645 kj/mol and the standard reaction gibbs energy is -5798 kj/mol. assume ∆h does not change to estimate the additional non-expansion work that may be obtained by raising the temperature to blood temperature, 37o c. enter your answer in kj/mol to two significant figures and do not enter the units.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1


Chemistry, 23.06.2019 06:30, Liapis
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
At 298 k the standard enthalpy of combustion of sucrose is -5645 kj/mol and the standard reaction gi...
Questions in other subjects:

Mathematics, 22.06.2021 19:40




Mathematics, 22.06.2021 19:40

Mathematics, 22.06.2021 19:40




Chemistry, 22.06.2021 19:40

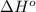 = -5645 kJ/mol
= -5645 kJ/mol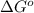 = -5798 kJ/mol
= -5798 kJ/mol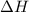 and
and 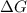 are as follows.
are as follows.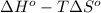
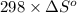
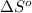 = 0.513 kJ/mol K
= 0.513 kJ/mol K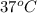 = (37 + 273) K = 310 K
= (37 + 273) K = 310 K