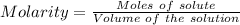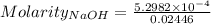Chemistry, 16.10.2019 01:30 jakails828
The concentration of a certain sodium hydroxide solution was determined by using the solution to titrate a sample of potassium hydrogen phthalate (abbreviated as khp). khp is an acid with one acidic hydrogen and a molar mass of 204.22 g/mol. in the titration, 24.46 ml of the sodium hydroxide solution was required to react with 0.1082 g khp. calculate the molarity of the sodium hydroxide.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
The concentration of a certain sodium hydroxide solution was determined by using the solution to tit...
Questions in other subjects:

History, 17.02.2020 04:15


History, 17.02.2020 04:15



Mathematics, 17.02.2020 04:23

Mathematics, 17.02.2020 04:23

Mathematics, 17.02.2020 04:23


Mathematics, 17.02.2020 04:24

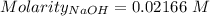
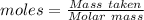
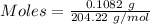
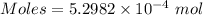
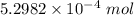 of KHP reacts with
of KHP reacts with