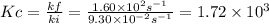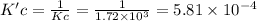At a given temperature, the elementary reaction a > b in the forward direction is first order in a with a rate constant of 1.60*10^2 s^-1. the reverse reaction is first order in b and the rate constant is 9.30*10^-2 s^-1what is the value of the equilibrium constant for the reaction a > b at this temperature? what is the value of equilibrium constant for the reaction b--> a at this temperature?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
At a given temperature, the elementary reaction a > b in the forward direction is first order in...
Questions in other subjects:

Mathematics, 12.12.2020 16:30

Social Studies, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

History, 12.12.2020 16:30


German, 12.12.2020 16:30


Social Studies, 12.12.2020 16:30