
Chemistry, 16.10.2019 02:00 jhonnysoriano9053
Constant‑boiling hcl can be used as a primary standard for acid–base titrations. a 50.00 ml sample of constant‑boiling hcl with a concentration of 0.1096 m was collected and titrated to an endpoint with 31.78 ml of a ba(oh)2 solution. what is the molarity of the ba(oh)2 solution

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 23:00, liv467
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
Constant‑boiling hcl can be used as a primary standard for acid–base titrations. a 50.00 ml sample o...
Questions in other subjects:

English, 15.12.2021 14:00


Social Studies, 15.12.2021 14:00




Health, 15.12.2021 14:00


Mathematics, 15.12.2021 14:00

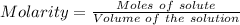
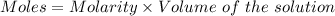
 :
:





