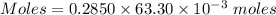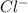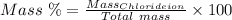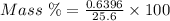Chemistry, 16.10.2019 00:00 Greghairston4839
The mass percent of cl⁻ in a seawater sample is determined by titrating 25.00 ml of seawater with agno₃ solution, causing a precipitation reaction. an indicator is used to detect the end point, which occurs when free ag⁺ ion is present in solution after all the cl⁻ has reacted. if 63.30 ml of 0.2850 m agno₃ is required to reach the end point, what is the mass percent of cl⁻ in the seawater (d of seawater = 1.024 g/ml)?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, hayleyconsole
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3


Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 04:00, Ezekielcassese
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
The mass percent of cl⁻ in a seawater sample is determined by titrating 25.00 ml of seawater with ag...
Questions in other subjects:


Mathematics, 30.07.2019 02:30


English, 30.07.2019 02:30

History, 30.07.2019 02:30

English, 30.07.2019 02:30

Biology, 30.07.2019 02:30

Social Studies, 30.07.2019 02:30


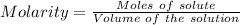
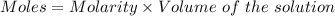
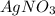 :
: