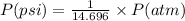Chemistry, 15.10.2019 20:20 rissaroo159
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the tire is 860 ml , that it is filled to a total pressure of 120 psi , and that the temperature is 26 ∘c. also, assume an average molar mass for air of 28.8 g/mol.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 10:30, sbelgirl2000
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
You know the right answer?
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the ti...
Questions in other subjects: