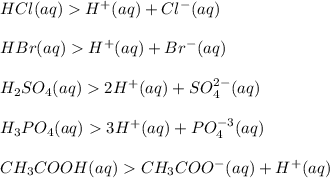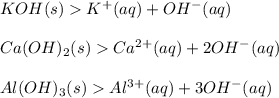Michael analyzes a chemical and determines it is calcium chloride (cacl). after further tests, michael declares that calcium chloride is a salt and has no effect on ph.
a. this means that the chemical will release:
(a) only h= ions into water.
(b) only oh - ions into water.
(c) equal amounts of oh- and h+ ions into water.
(d) neither oh- or h+ ions into water.
b. when placed in water, wha ions does this chemical release?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Michael analyzes a chemical and determines it is calcium chloride (cacl). after further tests, micha...
Questions in other subjects:

History, 27.10.2021 02:20

Mathematics, 27.10.2021 02:20

Chemistry, 27.10.2021 02:20



English, 27.10.2021 02:20




English, 27.10.2021 02:20

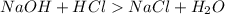
 is formed from a strong base
is formed from a strong base 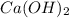 and strong acid HCl as shown in the Equation
and strong acid HCl as shown in the Equation
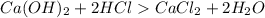
 and
and 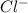 ions into water
ions into water
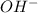 or
or  ions into water.
ions into water.