
Chemistry, 15.10.2019 04:30 smmailloux2335
Consider a reactant with an order of 1: what effect does that reactant have on the rate equation when the concentration is doubled 1) the reaction rate is double with respect to that reactant 2) the order doesnt effect the reaction so there will be no change 3) the reaction rate quadruples with respect to that reactant. 4) the reaction rate isn't changed by individual reactants so there will be no change.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2


Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 04:00, hailey200127
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Consider a reactant with an order of 1: what effect does that reactant have on the rate equation wh...
Questions in other subjects:

Spanish, 21.07.2019 09:50

Social Studies, 21.07.2019 09:50


History, 21.07.2019 09:50



English, 21.07.2019 09:50

Mathematics, 21.07.2019 09:50

Chemistry, 21.07.2019 09:50

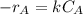
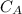 power is one (order 1), this could be proved just by checking it out in the rate law.
power is one (order 1), this could be proved just by checking it out in the rate law.

