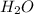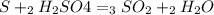Chemistry, 15.10.2019 04:00 johnnyboy41706
For each of the following unbalanced equations, calculate how many grams of each product would be produced by complete reaction of 10.8 g of the second reactant. (a) s(s) + h2so4(aq) → so2(g) + h2o(l) so2 webassign will check your answer for the correct number of significant figures. g h2o webassign will check your answer for the correct number of significant figures. g

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
For each of the following unbalanced equations, calculate how many grams of each product would be pr...
Questions in other subjects:




History, 31.10.2020 19:20



Social Studies, 31.10.2020 19:20


English, 31.10.2020 19:20

History, 31.10.2020 19:20

 of
of 
 of
of