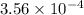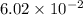Ammonia can be produced via the chemicalreaction
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2 nh_3 (g)
during the production process, the production engineer determinesthe reaction quotient to be q_2 = 3.56ã10â4. ifk_2 = 6.02ã10â2, what can besaid about the reaction?
1)the reaction has reached equilibrium.
2)the reaction is not at equilibrium and willproceed to the left.
3)the reaction is not at equilibrium and willproceed to the right.
4)the reaction is not at equilibrium, but it isnot possible to determine whether the reaction needs to proceedright or left to reach equilibrium.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, MrSavannahCat
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
You know the right answer?
Ammonia can be produced via the chemicalreaction
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2...
\rm n_2 (g) + 3 h_2 (g) \rightleftharpoons 2...
Questions in other subjects:


Mathematics, 14.06.2020 01:57



Mathematics, 14.06.2020 01:57