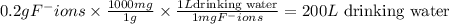Chemistry, 14.10.2019 23:30 anastasiakonni1
Fluoride ion is poisonous in relatively low amounts: 0.2 g of f− per 70 kg of body weight can cause death. nevertheless, in order to prevent tooth decay, f− ions are added to drinking water at a concentration of 1 mg of f− ion per l of water.
how many liters of fluoridated drinking water would a 70−kg person have to consume in one day to reach this toxic level?
answer in scientific notation

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Fluoride ion is poisonous in relatively low amounts: 0.2 g of f− per 70 kg of body weight can cause...
Questions in other subjects:








Mathematics, 13.03.2020 19:05


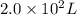 liters of fluoridated drinking water would a 70−kg person have to consume in one day to reach this toxic level
liters of fluoridated drinking water would a 70−kg person have to consume in one day to reach this toxic level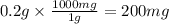 is toxic for 70kg person
is toxic for 70kg person