
Chemistry, 14.10.2019 22:10 foriegnngal
At 900 k the following reaction has kp=0.345: 2so2(g)+o2(g)ââ2so3(g) in an equilibrium mixture the partial pressures of so2 and o2 are 0.125 atm and 0.470 atm , respectively.
what is the equilibrium partial pressure of so3 in the mixture?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
You know the right answer?
At 900 k the following reaction has kp=0.345: 2so2(g)+o2(g)ââ2so3(g) in an equilibrium mixture the...
Questions in other subjects:








Computers and Technology, 07.09.2019 00:30


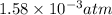
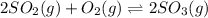 The expression for equilibrium constant for this reaction will be,
The expression for equilibrium constant for this reaction will be,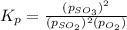
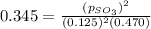
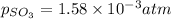
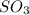 in the mixture is
in the mixture is 

