Chemistry, 14.10.2019 21:30 Affousietta
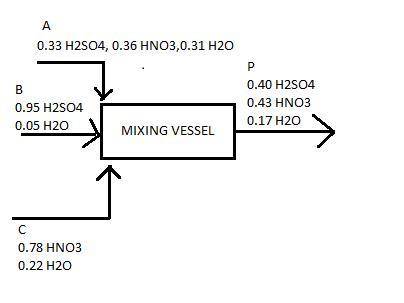
The spent acid from a nitration process contains 33% h2so4, 36% hno3 and 31% h2o. this is to be strengthened by the addition of concentrated h2so4 containing 95% h2so4 and nitric acid containing 78% hno3. the final strengths of mixed acid solution contains 40% h2so4 43% hno3 and the rest water. calculate : a. the amount of spent acid
b. the amount of concentrate h2so4
c. thr amount of concentrate hno3 to be mixed to produce 1500 kg of tge desired acid

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
The spent acid from a nitration process contains 33% h2so4, 36% hno3 and 31% h2o. this is to be stre...
Questions in other subjects:

Mathematics, 01.11.2020 16:30



Chemistry, 01.11.2020 16:30

Chemistry, 01.11.2020 16:40



Chemistry, 01.11.2020 16:40


Mathematics, 01.11.2020 16:40