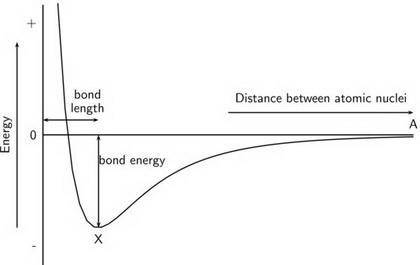
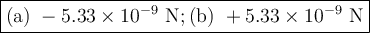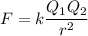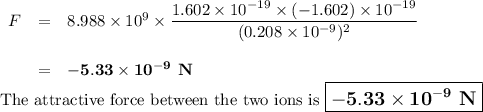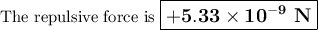Chemistry, 14.10.2019 22:00 tylrmannon
The atomic radii of li+ and 02 ions are 0.068 and 0.140 nm, respectively. (a) calculate the force of attraction between these two ions at their equilibrium interionic separation (i. e., when the ions just touch one another). (b) what is the force of repulsion at this same separation distance? 2.19 for a k+-c- ion pair, attractive and repulsive energies ea and er, respectively, depend on the distance between the ions r, according to

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
The atomic radii of li+ and 02 ions are 0.068 and 0.140 nm, respectively. (a) calculate the force of...
Questions in other subjects:

Biology, 26.02.2021 23:40




Mathematics, 26.02.2021 23:40


Mathematics, 26.02.2021 23:50

Biology, 26.02.2021 23:50

Mathematics, 26.02.2021 23:50

Mathematics, 26.02.2021 23:50