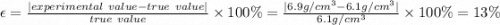Chemistry, 14.10.2019 22:00 yurlgurllmay
in a laboratory activity, the density of a sample of vanadium is determined to be 6.9 g/cm3 at room temperature. what is the percent error for the determined value?
a)
0.15%
b)
0.87%
c)
13%
d)
15%

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 06:10, ridzrana02
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1
You know the right answer?
in a laboratory activity, the density of a sample of vanadium is determined to be 6.9 g/cm3 at room...
Questions in other subjects:

Physics, 21.09.2019 21:30



Social Studies, 21.09.2019 21:30


Chemistry, 21.09.2019 21:30

Business, 21.09.2019 21:30


Mathematics, 21.09.2019 21:30

Physics, 21.09.2019 21:30