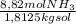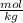Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
What is the molality of 150.0 g of nh3 dissolved in a solution that is 1.25 liters? the density of...
Questions in other subjects:

Computers and Technology, 01.09.2019 01:00


Mathematics, 01.09.2019 01:00


Mathematics, 01.09.2019 01:00

History, 01.09.2019 01:00


Mathematics, 01.09.2019 01:00

Mathematics, 01.09.2019 01:00

Social Studies, 01.09.2019 01:00

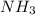 present in the 150 g.
present in the 150 g.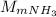 : Molar mass of
: Molar mass of 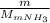
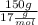
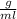
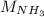 =
=