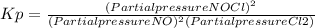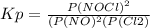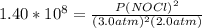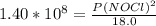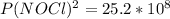Chemistry, 11.10.2019 17:30 BatmanVS1944
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no and 2.0 atm of cl2(g). what is the pressure of no(g) when equilibrium is reached?
3.6 × 10-4 atm
3.8 × 10-8 atm
0.5 atm
2.0 atm
1.0 atm
1.1 × 10-7 atm

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
2 no(g) + cl2(g) ⇄ 2 nocl(g) kp = 1.40 × 108 a reaction vessel initially contains 3.0 atm of no...
Questions in other subjects:

Mathematics, 17.12.2020 23:00

Business, 17.12.2020 23:00





Mathematics, 17.12.2020 23:00

Mathematics, 17.12.2020 23:00


Social Studies, 17.12.2020 23:00