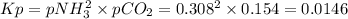nh4co2nh2< > 2nh3(g) + co2(g)


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3


Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Ammonium carbonate nh4co2nh2 decomposes as follows
nh4co2nh2< > 2nh3(g) + co2(g)
nh4co2nh2< > 2nh3(g) + co2(g)
Questions in other subjects:





English, 31.05.2021 02:00

Spanish, 31.05.2021 02:00




Mathematics, 31.05.2021 02:00