
Chemistry, 11.10.2019 03:30 mdndndndj7365
Consider the following reaction and select the false statement below.
10 i−(aq) + 16 h+(aq) + 2 mno4−(aq) → 5 i2(s) + 2 mn2+(aq) + 8 h2o(l)
(a) this is a reduction-oxidation (redox) reaction
(b) the iodine ion is the reducing agent
(c) the permanganate ion is the oxidizing agent
(d) the hydrogen ion is neither reduced nor oxidized
(e) in the reaction as written, ten moles of electrons are transferred from the oxidizing agent to the reducing agent

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Consider the following reaction and select the false statement below.
10 i−(aq) + 16 h+(aq) +...
10 i−(aq) + 16 h+(aq) +...
Questions in other subjects:



Chemistry, 28.10.2019 20:31

History, 28.10.2019 20:31

Mathematics, 28.10.2019 20:31

Mathematics, 28.10.2019 20:31


Arts, 28.10.2019 20:31

Mathematics, 28.10.2019 20:31

Spanish, 28.10.2019 20:31

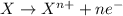
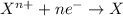
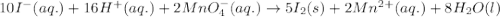
 ( × 5)
( × 5)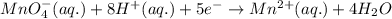 ( × 2)
( × 2)

