
Chromium has an atomic mass of 51.9961 u51.9961 u and consists of four isotopes, cr50,cr50, cr52,cr52, cr53,cr53, and cr54.cr54. the cr52cr52 isotope has a natural abundance of 83.79%83.79% and an atomic mass of 51.9405 u.51.9405 u. the cr54cr54 isotope has a natural abundance of 2.37%2.37% and an atomic mass of 53.9389 u.53.9389 u. the natural abundances of the cr50cr50 and cr53cr53 isotopes exist in a ratio of 0.4579: 1,0.4579: 1, and the cr50cr50 isotope has an atomic mass of 49.9460 u.49.9460 u. determine the atomic mass of the cr53 isotope. cr53 isotope.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2



Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Chromium has an atomic mass of 51.9961 u51.9961 u and consists of four isotopes, cr50,cr50, cr52,cr5...
Questions in other subjects:

History, 30.07.2019 15:30








Chemistry, 30.07.2019 15:30

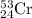 isotope is 52.8367 amu
isotope is 52.8367 amu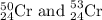 isotopes =
isotopes = ![[100-(83.79+2.37)]=13.84\%](/tpl/images/0309/0133/29a15.png)
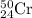 isotope =
isotope = 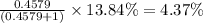
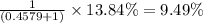
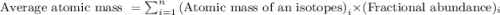 .....(1)
.....(1)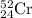 isotope:
isotope: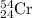 isotope:
isotope:![51.9961=[(49.9460\times 0.0437)+(51.9405\times 0.8379)+(x\times 0.0949)+(53.9389\times 0.0237)]\\\\x=52.8367amu](/tpl/images/0309/0133/822e6.png)


